Abstract
This Review paper gives a brief introduction about gas sensors, its types, their construction and their applications. The different types of gas sensors explained include metal oxide-based gas sensors, optical gas sensors, electrochemical gas sensors, Infrared gas sensors, Calorimetric gas sensors, Acoustic Based gas sensors. All these sensors have been used for the detection of hazardous gases with improvised sensitivity and selectivity.
1. Introduction
A sensor is a device that provides usable output in response to a specified measure by acquiring a physical quantity and converting it into a signal suitable for processing. One of these is a gas sensor that detects the presence of gas in that particular area, often used as a part of a safety system, where it detects a signal physical condition and chemical compounds. As the air surrounding us contains different amount of gases which could be hazardous to human health, atmospheric pollutants or of significance to an industrial or medical process, It becomes therefore very imperative to detect the presence of these gases since the environment we dwell in consists of humans, plants and animals as its main inhabitants, so the safety of their lives is of topmost priority.

Based on the concentration of gas the sensor detects in the atmosphere, corresponding potential difference is produced by it by changing the resistance of the material present within it, which further measure the output voltage. Based on this voltage type of gas and its concentration can be estimated. The sensing factor majorly depends upon the type of sensing material present inside it. Generally, these sensors consists of comparators set at particular value of threshold which determine the change in concentration. Depending on factors like selectivity, detection limit, response time, size, low power consumption and their capability of being wireless prioritise them.
2. Different types of Gas sensors
In this section a review on the different types of sensors and their principle of operation will be discussed.
2.1 Metal oxide Based Gas Sensor
These sensors are devices made up of heated metal oxide which are used for the measurement of the gas concentration of a target gas by measuring the electrical resistance of the device. They work on the principle of the reversible gas adsorption process at the surface of the heated oxide usually oxides of tin deposited on a silicon slice by a chemical vapour deposition method. Absorption of the sample gas on the oxide surface followed by catalytic oxidation results in a change of electrical resistance of the oxide material which is then related to the sample gas concentration which is monitored by the meter as shown in Fig. 2. The heater at the base is used for heating up the sensor to a constant temperature of about 200-250 °C so as to speed up the reaction rate.

oxide gas sensor.
Normally the atmosphere will contain more oxygen than combustible gases. The oxygen particles attract the free electrons present in SnO2 which pushes them to the surface of the SnO2. As there are no free electrons available output current will be zero. When the sensor is placed in the toxic or combustible gases environment, this reducing gas reacts with the adsorbed oxygen particles and breaks the chemical bond between oxygen and free electrons thus releasing the free electrons. As the free electrons are back to their initial position they can now conduct current, this conduction will be proportional to the amount of free electrons available in SnO2, if the gas is highly toxic more free electrons will be available.
Advantage: Mechanically robust, works well at constant high humidity condition.
Disadvantage: Susceptible to contaminants and changes due to environmental conditions. Non- linear response effects complexity.
2.2 Optical Gas Sensor
An optical gas sensor comprises a light-emitting element; a photo-detecting element; a gas sensing element including a thin film containing an organic pigment, the gas sensing element responding to light emitted from the light-emitting element to emit fluorescence or phosphorescence; and a filter for selectively picking up fluorescence or phosphorescence, the gas sensing element and the filter being disposed in an optical path ranging from the light-emitting element to the photo-detecting element.
The optical gas sensor can sense a gas to be sensed by measuring a reversible change of colour or a reversible change of intensity of fluorescence or phosphorescence of the thin film containing an organic pigment, which results from the contact of the thin film with the gas. To be more specific, an exciting light emitted from the light-emitting element reaches the organic pigment contained in the thin film of the gas sensing element. The exciting light excites the organic pigment to cause it to emit fluorescence or phosphorescence. In this case, when the thin film contacts the gas, the wavelengths of the absorption spectrum of lights emitted from the gas sensing element reversely vary or the intensity of the fluorescence or phosphorescence reversely varies.
The light from the gas sensing element is applied to the photo-detecting element, through the filter for separating the fluorescence or phosphorescence from the exciting light. The reversible colour variation of the exciting light from the thin film or the intensity variation of the fluorescence or phosphorescence emitted from the thin film is measured by the photo-detecting element. In this way, the gas is sensed.
Advantage: Easy to operate in absence of oxygen. Not affected by electromagnetic interference. The monitoring area is very wide.
Disadvantage: Affected by ambient light interference.
2.3 Electrochemical Gas Sensor
These types of sensors allow gases to diffuse through a porous membrane to an electrode where it is either reduced or oxidized at the electrode.
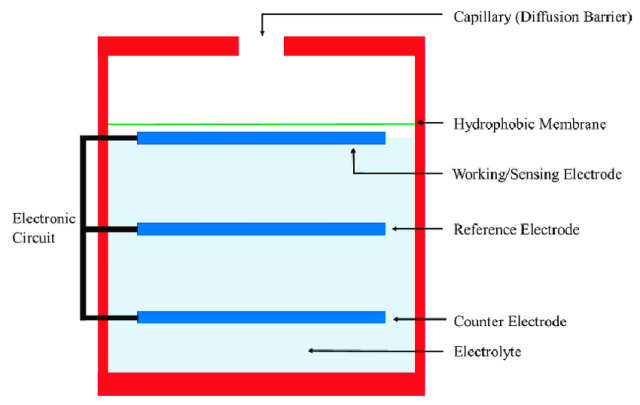
Electrochemical sensors operate by reacting with a target gas and producing an electrical signal that is proportional to the gas concentration. A typical electrochemical gas sensor consists of a sensing electrode or working electrode and a counter electrode which are separated by a thin layer of electrolyte. Before the gas comes in contact with the sensor, it goes through a thin capillary-type opening and then diffuses through a hydrophobic barrier before finally reaching the electrode surface. The function of this membrane is to prevent liquid electrolytes from leaking out and generate enough electrical signals at the sensing electrode. It also consists of a reference electrode whose function is to maintain a stable and constant potential at the sensing electrode due to the continuous electrochemical reactions occurring on the electrode surface. The electrochemical reaction with the target gas generates a flow of current flow between the sensing and counter electrodes. The electrolyte is responsible for carrying the ionic charges across the electrode.
Advantage: Measures toxic gases in relatively low concentrations. A wide range of gases can be detected.
Disadvantage: Failures modes are unrevealed unless advanced monitoring techniques used.
2.3 Infrared Gas Sensor
Infrared sensors consist of a detector that converts electromagnetic radiation energy into electrical signals. The Detectors are of different types namely: Thermoelectric, Thermistor Bolometer, Pyroelectric detector and a Photon detector. It also consists of an infrared source which could be a regular incandescent light or a heated wire filament which can be used for the detection of CO2, CO and other hydrocarbons. Another component is an optical fibre which could be of two types: dispersive and non-dispersive. Non-dispersive types use discrete optical bandpass filters and are mostly used for gas sensor applications while the dispersive types use an optical device like a grating or prism. The last but not the least is the gas cell which allows the light path so as to interact with the target gas. Infrared gas sensors are used for detecting different gases like methane, ethane, propane, butane, benzene toluene, xylene and other alcohols like methanol, ethanol etc. [3].
Advantage: Uses only physical technique. No unseen failure modes. Can be used in inert atmospheres.
Disadvantage: Not all gases have IR absorption. Sequential monitoring is slower on multi-point analysers and also more user expertise required.
2.4 Calorimetric Gas Sensor
Calorimetric sensors are based on the measurement of the heat produced by the molecular recognition reaction and the amount of heat produced is correlated to the reactant concentration. The principle of the calorimetric sensors is the determination of the presence or concentration of chemical species by the measurement of the enthalpy change produced by any chemical reaction or physisorption process that releases or absorbs heat.
Calorimetric sensors or chemoresistors can be classified into low-temperature chemoresistors and high-temperature chemoresistors. Low-temperature chemoresistors consist of chemically sensitive layers applied over interdigitated electrodes on an insulating substrate. These types of chemoresistors are used in the detection of ethanol, methanol and other organic volatile molecules. High-temperature chemoresistors consist of micromachined semiconductor hotplates with a sensing film on a thermally insulated inorganic membrane. ZnO, InO, GaO, SnO are usually employed as sensitive materials. Gaseous electron donors (H) or electron acceptors (NOx) adsorb on metal oxides and form surface states, which can exchange electrons with the semiconductor.
Advantage: Robust but simple construction. Easy to operate in absence of oxygen. The measuring range is very wide.
Disadvantage: Reaction due to heating wire.
2.5 Acoustic Based Gas Sensor
Acoustic wave sensors are so named because their detection mechanism is a mechanical, or acoustic wave. As the acoustic wave propagates through or on the surface of the material, any changes to the characteristics of the propagation path affect the velocity and/or amplitude of the wave. Changes in velocity can be monitored by measuring the frequency or phase characteristics of the sensor and can then be correlated to the corresponding physical quantity being measured. An acoustic wave sensor contains a receptor which is an element that is sensitive to an analyte and a transducer i.e. an element that converts the response into an electrical signal.
There are different types of acoustic wave sensors which are based on the type of wave propagation. Acoustic wave sensors have a variety of applications as in temperature, pressure, mass, chemical etc. The principle of operation of the acoustic chemical sensor is described as follows. When a receptor film is introduced onto the vibrating surface of a transducer that is activated by an electronic device, the characteristics of the receptor film such as its mass and thickness are changed when exposed to an analyte. This change directly affects the vibration frequency, amplitude and phase. The shift is directly proportional to the analyte concentration.
Advantage: Detect nerve and blister agents Battery-less and could be used for wireless applications. Could be placed in harsh and rotating parts.
Disadvantage: Due to its small size there is difficulty in handling during the fabrication process.
3 Application of Gas Sensors
- Used in industries to monitor the concentration of the toxic gases. Used in households to detect an emergency incidents.
- Used at oil rig locations to monitor the concentration of the gases those are released.
- Used at hotels to avoid customers from smoking.
- Used in air quality check at offices.
- Used in air conditioners to monitor the CO2 levels.
- Used in detecting fire.
- Used to check concentration of gases in mines.
- Used in Breath analyzer.
Reference
- [ 1 ] [Online]. Available:http://engineering.nyu.edu/gk12/amps- cbri/pdf/Intro%20to%20Sensors.pdf.
- [ 2 ] F. Bagheri. [Online]. Available: https://www.powershow.com/view4/7b13b 9-ZjExN/Gas_Sensors_powerpoint_ppt_presen tation.
- [ 3 ] M. N. H. K. A. Zainab Yunusa, “Research Gate,” [Online]. Available: https://www.researchgate.net/publication/285988329_Gas_Sensors_A_Review
- [ 4 ] [Online]. Available: https://components101.com/articles/introd uction-to-gas-sensors-types-working-and- applications#:~:text=Gas%20Sensor%20Cons truction.%20Of%20all%20the%20above- listed%20types%2C,the%20following%20part s.%20Gas%20sensing%20layer.%20Heater%2 0Coil
- [ 5 ] “Researc Gate,” [Online]. Available: https://www.researchgate.net/figure/Schematic-representation-of-a-metal-oxide-TiO2-based-gas-sensor-for-SOx-detection_fig1_321317592
- [ 6 ] I. A. F. S. Pu, “Google Patents,” [Online]. Available: https://patents.google.com/patent/US5030009
- [ 7 ] “Research Gate,” [Online]. Available: https://www.bing.com/images/search?view= detailV2&ccid=A9G6uChE&id=5031F77758FE 846854D4E509CD7C8F15968AD2E3&thid=OI P.A9G6uChEiOPV3KZSTi81NgHaEs&mediaurl =https%3a%2f%2fwww.researchgate.net%2f publication%2f342722833%2ffigure%2fdown load%2ffig1%2fAS%3a91047632
- [ 8 ] [Online]. Available: https://transducersensors.com/calorimetric- sensors/
Here is interesting article for you: Industrial off-gases as raw material in cosmetic industry








